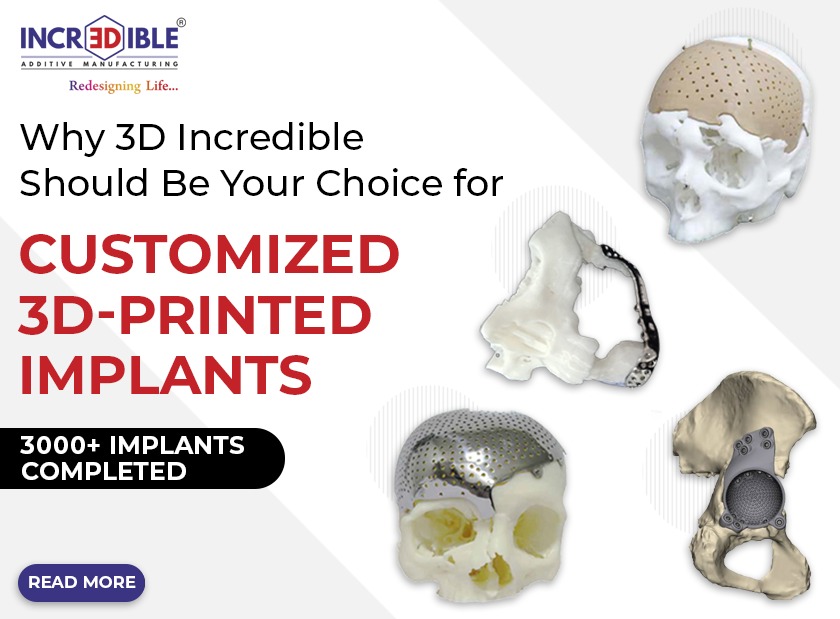
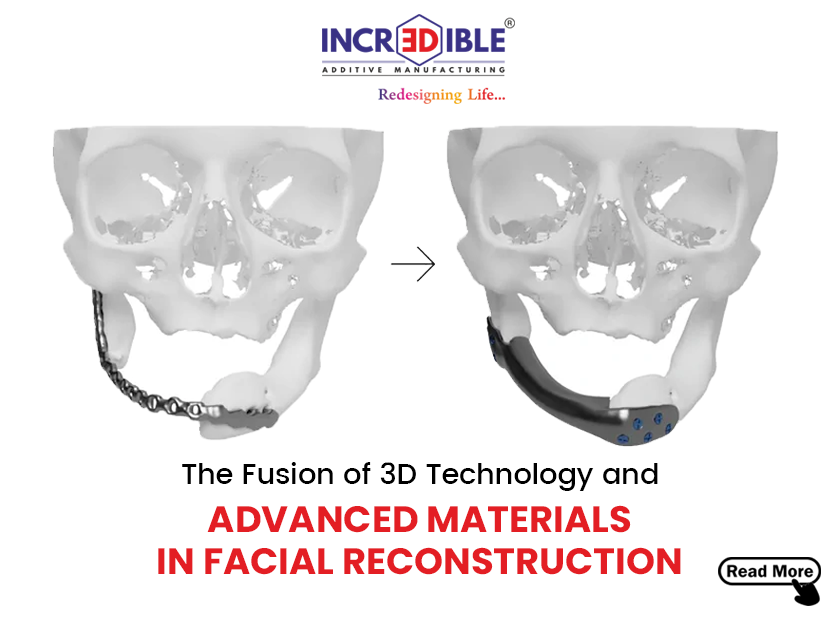
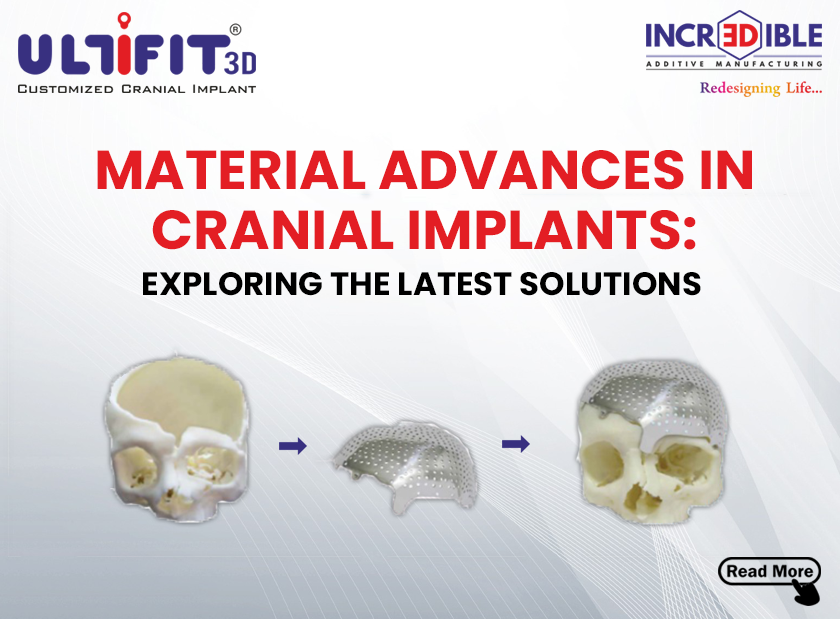
What are FDA-approved Software and How Are They Useful in Implant Manufacturing?
The design and production of implants have advanced significantly in the constantly changing world of medical technology. The incorporation of software into the manufacturing process has been crucial to this development. Particularly, FDA-approved (Food and Drug Administration) software assures that these technological advancements adhere to strict safety and efficacy standards. This article delves into the world of FDA-approved software, examining what it is and how it has changed the manufacturing of implants.
What is FDA-Approved Software?
As a regulatory body, the FDA is crucial in ensuring the efficacy and safety of medical devices, including implantable devices. The term FDA-approved software refers to computer programs or applications that have passed the exacting standards established by the FDA and are utilized in the design, production, and quality control processes of medical implants. These requirements include precision, dependability and security, which are crucial in bio-implants.
The Significance of FDA-approved Software in Implant Manufacturing
Precision Engineering: With FDA-approved software, manufacturers can produce intricate implant designs and models with unmatched precision. This precision is essential in orthopedic and dental implants, where fit and functionality directly affect the quality of life.
Customization: Each patient’s anatomy is unique. Implants designed using FDA-approved software replicate the precise features of a patient’s anatomy. This ensures that patient-specific implants match the individual characteristics of each patient, whether it’s a dental crown or a knee replacement. Customization guarantees a perfect fit and optimal performance.
Simulation and Testing: Modern software tools enable virtual environment simulations and testing, allowing for the evaluation of various implant designs in terms of performance, stress points, and durability. This approach reduces the time and costs associated with traditional trial-and-error methods.
Streamlined Production: The manufacturing process is made more efficient by FDA-approved software. It facilitates automation, enabling effective production with strict quality control. As a result, implants are produced more quickly without sacrificing quality.
Data Analysis: These software packages often include data analysis features. After implants are in use, manufacturers can collect information about their performance. This real-world feedback loop enables continuous design and material improvements, resulting in better and more durable future implants.
Compliance and Documentation: The FDA approval procedure ensures that the software complies with legal requirements. This means that the manufacturer thoroughly documents the software’s functionality and safety precautions, which is essential for audits and compliance checks.
Conclusion
FDA-approved software has become essential for the production of modern implants. By harnessing the latest computational tools, manufacturers such as Incredible AM Pvt Ltd in Pune can design safe and reliable patient-specific implants. As a pioneer in 3D printing in India, Incredible AM Pvt Ltd adheres to stringent evaluation and approval procedures used by regulatory organizations like the FDA to ensure that these software solutions meet the highest standards. With our 3D-printed implants, designed using FDA-approved software, patients can expect safer procedures, quicker recovery times, and implants that genuinely enhance their quality of life.